Revolutionizing Point-of-Care Diagnostics for Traumatic Brain Injury (TBI)
Overview
Digital Health Solutions partnered with a medical diagnostics company to develop an innovative point-of-care (POC) system for detecting mild traumatic brain injury (TBI). This cutting-edge solution integrates nanotechnology, mobile devices, and a cloud platform to deliver near-instant, accurate results directly at the patient’s side. Designed for deployment in ERs, urgent care centers, and nursing homes, the system comprises a Base Station, Handheld Unit, single-use cartridges, and a secure cloud backend. Our team led the entire development lifecycle—from system design and development to regulatory alignment and cybersecurity hardening.
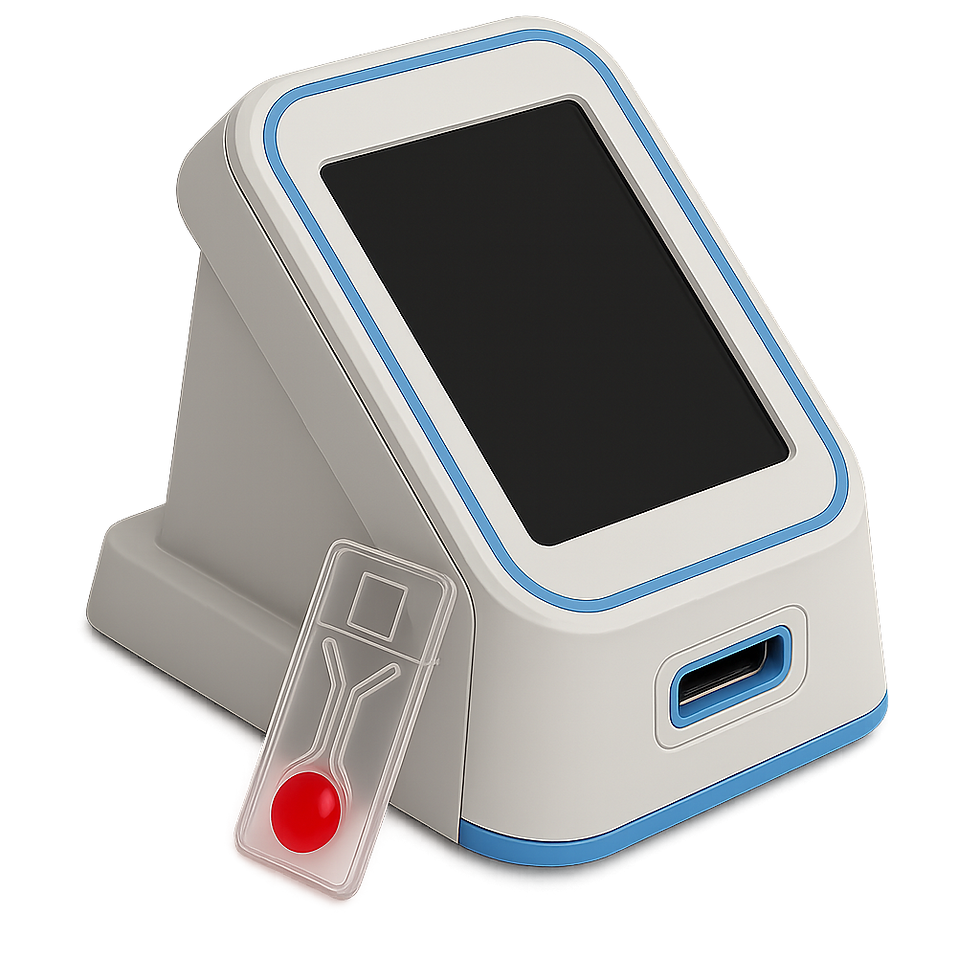
Project Highlights
Challenge
Ultra-Fast Diagnostic Requirements: Delivering reliable test results within two minutes from sample collection.
Hardware & Software Integration: Seamlessly connecting Base Station, Handheld Unit, cartridges, and cloud services.
Data Privacy & Security: Meeting rigorous cybersecurity and patient data protection standards.
Scalability: Developing a scalable cloud infrastructure to support device registration, data storage, and user management.
Regulatory Complexity: Ensuring compliance with FDA and IEC 62304 Class C requirements for in-vitro diagnostics.
Our Approach
Systems Engineering Excellence: Defined full user/system/subsystem requirements with detailed traceability and risk controls.
Robust Software & Firmware Development: Built advanced firmware for rapid biomarker analysis and a secure Android app for clinicians.
Cloud Infrastructure & Security: Implemented Azure IoT-based cloud with PKI-secured communication and data workflows.
Full Lifecycle Compliance: Delivered V&V, SDLC, cybersecurity documentation, and QMS integration in alignment with FDA expectations.
Impact
Commercialization-Ready Platform: Positioned the TBI system for FDA submission and market launch.
Real-Time Results: Enabled clinicians to make faster, data-driven treatment decisions at the point of care.
System-wide Integration: Delivered a seamless user experience through connected hardware, app, and cloud systems.
Regulatory & Cybersecurity Leadership: Ensured secure data handling, passed third-party penetration testing, and established post-market surveillance protocols.
Takeaways
Full-Stack Technical Ownership: Delivered end-to-end development — from embedded firmware to mobile and cloud.
Regulatory and Process Maturity: Aligned with FDA, IEC 62304, and enhanced documentation expectations, with validated tools and test processes.
Cybersecurity Leadership: Proactively addressed FDA cybersecurity guidance through threat modeling, PKI infrastructure, and vulnerability testing.
Collaborative, Agile Delivery: Maintained close alignment with client teams to meet aggressive timelines and achieve compliance milestones.